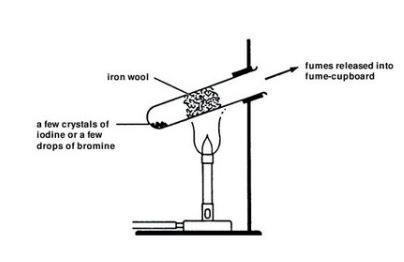
It clearly cannot be sulfite or sulphur dioxide because in the presence of water these immediately react with iodine to make sulphuric acid and hydroiodic acid. You have to perform this reaction outside or in a fumehood.

Classic Chemistry Colorize Colorless Liquids With Black Magic Aka The Iodine Clock Reaction Science Experiments Wonderhowto
Now if you meant to ask what will happen if you mix sulphuric acid and iodide I1- then that is a different story.
Iodine react with tin and sulphuric acid. They are oxidised to iodine by the concentrated sulphuric acid. Probably dissolve the iodine so the mess will become somewhat purple in color. At this point it will start producing toxic gases including hydrogen iodide sulfur dioxide hydrogen sulfide and vaporized sulfuric acid.
Iodine reacts with concentrated nitric acid according to the following equation. The equilibrium constants of the reactions producing these compounds are determined. Iodine is a useful chemical for basic chemistry reactions a disinfectant a histological stain and an identification method for starches.
This SI process is a chemical heat engine. Original post by Phalange Im doing Edexcel AS Chemistry new spec. To this a solution containing potassium iodide sodium thiosulfate and starch is added.
Cool cautiously dilute with water add 65 ml of concentrated sulphuric acid cool again and dilute with water to 500 ml in a volumetric flask. Sulphuric acid is a VERY Strong Oxidising agent that means that it will oxidise your Tin therefore we get textSnrightarrow textSn22text e- in the second part I think you have reacted your 2 electrons formed in the previous reaction with Hydrogen ion But I doubt this part. In the first slow reaction iodine is produced.
Hydrogen ions H are used as a catalyst for the reactants and are disassociated from Sulphuric acid. If you have remembered from early reaction with metal chloride HCl cant reduces H2SO4 further whereas HBr can. I do not have any sulfuric acid and it is expensive to purchase I do have a large supply of sodium bisulfate.
This solution contains 200 μg Sn per ml. Probably dissolve the iodine so the mess will become somewhat purple in color. The reduction of the sulphuric acid is more complicated than before.
We get The product called s feso4 that is iin sulfate. Hot and concentrated nitric acid is a powerful oxidising agent. 2 H 2 O 2 H 2 O 2.
So the sulphur is oxidised. Again Sulphuric acid reacts with Metal iodide to form HI. Now if you meant to ask what will happen if you mix sulphuric acid and iodide I 1- then that is a different story.
As a result of the reaction of sulfuric acid H 2 SO 4 and potassium iodide KI produces hydrogen sulfide H 2 S water H 2 O iodine I 2 potassium sulfate K 2 SO 4 H 2 SO 4. Unfortunately its use as an illicit drug precursor has made. During the reaction the iodine solution turns from a dark brown colour into a colourless solution this is because the iodine is reacting to produce the iodopropanone and the the colourless iodine ions.
This element has been known to humanity since ancient times and was considered to be one of the rarest and most valuable metals so items made from tin could only be afforded by the richest inhabitants of the Roman Empire and Ancient Greece. What happens when hot concentrated nitric acid is poured on powdered Sulphur. This question is the salt obtained when a chemical change takes place on audition of iron to dilute sulfuric acid.
4Ch ¼ 1 k þ Cð5ÞOne can conclude that the absorption of phosphonium iodine salt on nickel surface in 1 M sulphuric acid medium occurs according to the Langmuir adsorption isothermFrom the intercepts of the straight lines on the Ch axis k values could be calculated Table 2Furthermore the equilibrium constant k for the adsorption process of the inhibitor could also. This reaction produces also I 2 O which is not formed in the pure acid. All solutions were adjusted to pH 22 using sulphuric acid to ensure that iodine initially existed only as I 2.
5H 2 SO 4 8KI H 2 S 4H 2 O 4I 2 4K 2 SO 4. Iodide ions are stronger reducing agents than bromide ions are. The heaviest of the stable halogens it exists as a semi-lustrous non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius and boils to a violet gas at 184 degrees CelsiusThe element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later.
Report 11 years ago. This might not be solutions with chemical compositions relevant for an accident scenario but the aim of these experiments was to study the reactions between I 2 and metal surfaces not to do laboratory scale experiments on aqueous phase chemistry in the containment. I thought the reaction was simply oxidizing iodide to iodine with hydrogen peroxide so I dont understand the purpose of the acid.
Iodine is a chemical element with the symbol I and atomic number 53. Theres these reacitons of metal halides with sulphuric acid. The reaction dates back to 1814 when Colin and Gaultier de Claubry and independently Stromeyer introduced the iodine reaction for starch.
As you add it will produce iodine. Iodide is a strong enough reducing agent to reduce sulphuric acid. So in this question what are we going to do is we are going to add iron to dilute sulfuric acid.
Iodide is a strong enough reducing agent to reduce sulphuric acid. The sulfur and iodine compounds are recovered and reused hence the consideration of the process as a cycle. Heat enters the cycle in high-temperature endothermic chemical reactions 2 and 3 and heat exits the cycle in the low-temperature exothermic reaction 1.
A variant of the acidified iodine reaction appears to have been used. It also cannot be sulfide because in the presence of water this immediately reacts to form elemental sulphur and hydroiodic acid in fact this is an old-fashioned way of making HI by bubbling H2S through a suspension of iodine in. Tin is a light metal located in the 14th group of the periodic table with the atomic number of 50.
If you mix sulphuric acid with iodine I2 not much. Basically NaFNaCl has 1 reaction NaBr has 2 and NaI has 3 so Ill show NaI as it includes all others. The reactions of iodic acid with iodine in 96 sulfuric acid produce IOHSO 4 I 3 and I 5 just like in the 100 acid.
It calls for sulfuric acid but I dont see what this does do help the reaction. Sulphur gets oxidized to Sulphuric acid and nitrogen dioxide is produced. Second add concentrated sulfuric acid directly to the solution and constantly stir.
KI s H2SO4 l HI g KHSO4 s This is the primary reaction that would occur in every reactions of Concentrated sulfuric acid with Metal Halide. The iodide ions are powerful enough reducing agents to reduce it. There are two reactions occurring simultaneously in the solution.
Standard tin stock solution dissolve 0100 g of pure tin by evaporation to fumes with 20 ml of concentrated sulphuric acid. It can oxidise sulphur to sulphuric acid. NaI s H2SO4 aq ----- NaHSO4 s HI g.
What salt is formed when iron reacts with Sulphuric acid. This method starts with a solution of hydrogen peroxide and sulfuric acid. If you mix sulphuric acid with iodine I2 not much.
The historical development of the iodine-sulphuric acid reaction for amyloid is described.


